The global medical device testing market size was valued at USD 5.20 billion in 2024 and is expected to reach USD 11.82 billion by 2032, at a CAGR of 10.80% during the forecast period
Introduction
The medical device testing market plays a pivotal role in ensuring the safety, efficacy, and regulatory compliance of medical devices across the globe. Medical devices, ranging from diagnostic instruments and implantable devices to wearable technologies and surgical equipment, must undergo rigorous testing to meet international standards and safeguard patient health.
Medical device testing encompasses various processes, including biocompatibility testing, performance evaluation, electrical and mechanical testing, sterilization validation, and regulatory compliance testing. These processes ensure that medical devices function accurately, meet quality standards, and minimize risks to patients.
The market is witnessing significant growth due to increasing adoption of advanced medical devices, stringent regulatory requirements, rising demand for high-quality healthcare solutions, and advancements in testing technologies. This article provides an in-depth overview of the medical device testing market, including growth drivers, challenges, opportunities, segmentation, regional insights, competitive landscape, and future prospects.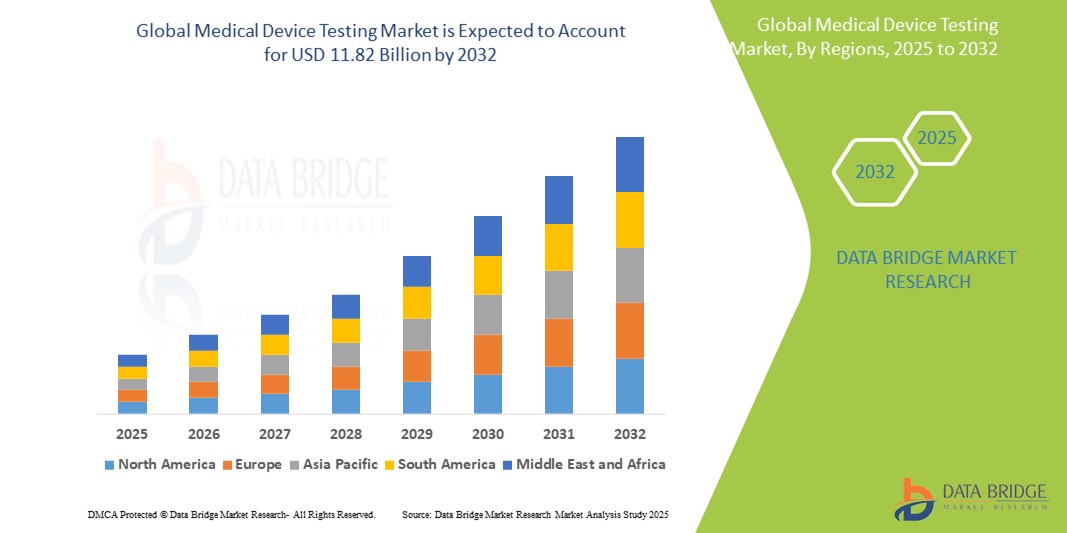
Market Overview
Medical device testing services are critical to the lifecycle of a medical device, from development and prototyping to post-market surveillance. The testing process helps manufacturers comply with guidelines established by regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), International Organization for Standardization (ISO), and International Electrotechnical Commission (IEC).
Testing is conducted in specialized laboratories or on-site facilities using advanced analytical instruments, simulation technologies, and automated testing systems. The scope of testing includes chemical analysis, mechanical durability testing, electrical safety, microbiological evaluation, and software validation, depending on the type of device.
The increasing complexity of medical devices, including digital and connected devices, wearable technologies, and implantables, has further fueled the need for specialized testing services. Manufacturers increasingly rely on third-party testing providers to ensure accuracy, compliance, and faster time-to-market.
Key Market Drivers
Rising demand for advanced and technologically sophisticated medical devices
Stringent regulatory requirements and safety standards imposed by global health authorities
Growing prevalence of chronic diseases and aging population requiring medical device interventions
Increasing outsourcing of testing services by device manufacturers to reduce costs and improve efficiency
Technological advancements in testing methodologies, automation, and simulation
Expansion of healthcare infrastructure in emerging markets driving the adoption of quality-assured devices
Market Challenges
High costs associated with comprehensive testing and validation processes
Regulatory variations across regions leading to complex compliance requirements
Rapid technological evolution of medical devices requiring constant updates in testing protocols
Limited availability of specialized testing laboratories in certain regions
Long turnaround times for certain complex tests affecting time-to-market
Opportunities in the Market
Growth of digital and connected medical devices requiring software and cybersecurity testing
Adoption of automated testing platforms to improve accuracy and reduce costs
Expansion into emerging markets with increasing healthcare infrastructure investments
Integration of AI and machine learning in testing to predict device performance and identify risks
Partnerships between testing providers and device manufacturers to offer end-to-end solutions
Increasing focus on post-market surveillance and real-world evidence testing
Segmentation Analysis
By Testing Type
Biocompatibility Testing
Performance and Functional Testing
Electrical and Mechanical Testing
Sterilization and Microbiological Testing
Software and Cybersecurity Testing
Regulatory Compliance Testing
By Device Type
Diagnostic Devices
Therapeutic and Surgical Devices
Implantable Devices
Wearable Devices
In Vitro Diagnostics
By End User
Medical Device Manufacturers
Contract Research Organizations (CROs)
Hospitals and Healthcare Facilities
Research and Academic Institutions
Regional Insights
North America
North America holds a dominant position in the medical device testing market due to the presence of advanced healthcare infrastructure, stringent regulatory frameworks, and high adoption of innovative medical technologies. The U.S. leads the region, supported by FDA guidelines, specialized testing laboratories, and increasing outsourcing by device manufacturers.
Europe
Europe demonstrates significant market growth, driven by regulatory mandates such as CE marking, ISO standards, and the Medical Device Regulation (MDR). Countries like Germany, France, and the UK are major contributors to the regional market due to the high presence of medical device manufacturers and testing service providers.
Asia-Pacific
Asia-Pacific is witnessing rapid growth owing to increasing healthcare expenditure, expansion of medical device manufacturing, and growing adoption of advanced healthcare technologies in countries such as China, India, Japan, and Australia. Emerging markets in the region are investing in establishing testing laboratories to meet global standards.
Middle East and Africa
The Middle East and Africa are gradually adopting medical device testing services, driven by healthcare infrastructure development, rising awareness of quality standards, and increasing imports of medical devices. However, adoption remains limited compared to developed regions.
Latin America
Latin America is experiencing steady growth due to expanding healthcare facilities, rising demand for advanced medical devices, and increasing regulatory initiatives in countries such as Brazil and Mexico.
Competitive Landscape
The medical device testing market is competitive, with key global and regional players focusing on technological innovation, strategic partnerships, and geographic expansion. Leading companies include:
SGS SA
Intertek Group plc
Eurofins Scientific
Bureau Veritas S.A.
TUV SUD AG
Nelson Laboratories, LLC
UL LLC
These companies invest in advanced testing technologies, automation, and laboratory expansion to enhance service offerings. Strategic collaborations with device manufacturers and research institutions are common approaches to strengthen market presence and ensure compliance with evolving regulations.
Future Outlook
The future of the medical device testing market is expected to be shaped by technological advancements, regulatory evolution, and the increasing complexity of medical devices. Digital and connected devices, wearable technologies, and AI-enabled healthcare solutions will drive demand for advanced testing methodologies.
Emerging markets offer substantial growth potential as healthcare infrastructure expands, awareness of quality standards increases, and local manufacturing of medical devices rises. The integration of automated testing, AI-driven predictive analytics, and software validation will further enhance accuracy, reduce costs, and improve the speed of device approval processes.
The market is also expected to witness growth in post-market surveillance testing, real-world evidence generation, and cybersecurity evaluation for connected devices, ensuring patient safety and long-term device reliability.
Conclusion
The medical device testing market is poised for significant growth, driven by increasing adoption of advanced medical technologies, stringent regulatory requirements, and growing awareness of device safety and efficacy. While challenges such as high costs, regulatory complexity, and evolving technology exist, innovation in testing methodologies, automation, and emerging markets presents substantial opportunities.
As the healthcare industry continues to evolve, medical device testing will play a crucial role in ensuring patient safety, regulatory compliance, and the overall quality of medical devices, making it an essential component of global healthcare delivery.
FAQs
What are the key types of medical device testing services available?
How is automation transforming medical device testing processes?
Which regions are leading in medical device testing adoption and infrastructure?
What challenges do manufacturers face in meeting global regulatory requirements?
How are companies innovating to ensure faster and more accurate testing of medical devices?
Equip yourself with actionable insights and trends from our complete Medical Device Testing Market analysis. Download now:https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
Browse More Reports:
Global Sexually Transmitted Diseases (STDs) Antimicrobial Medication Market
Global Shock Absorption Running Shoes Market
Global Sialolithiasis Treatment Market
Global Silica Based Matting Agents Market
Global Silicone Oil Market
Global Simultaneous Localization and Mapping Market
Global Single Loop Controller Market
Global Sinus Dilation Devices Market
Global Sixth Nerve Palsy Treatment Market
Global Skincare Packaging Market
Global Sludge Dewatering Equipment Market
Global Smart Connected Assets and Operations Market
Global Smart Diabetes Management Market
Global Snacks and Savoury Food Equipment Market
Global Social Mapping Management Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com









